Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Suzuki, Hideya; Tsubata, Yasuhiro; Kurosawa, Tatsuya; Shibata, Mitsunobu; Kawasaki, Tomohiro; Urabe, Shunichi*; Matsumura, Tatsuro
Analytical Sciences, 32(4), p.477 - 479, 2016/04
Times Cited Count:24 Percentile:68.53(Chemistry, Analytical)An impeccable, high-performance new reagent called alkyl diamide amine (ADAAM) was examined from the viewpoint of mutual separation of Am(III) and Eu(III). ADAAM has three donor atoms, one soft N-donor atom and two hard O-donor atoms, in the central frame. The combination of soft and hard atoms affords a tridentate donor set of atoms that ensures remarkable extractability and selectivity of Am(III) and Eu(III) in highly acidic media.
Kato, Tetsuya*; Inoue, Tadashi*; Iwai, Takashi; Arai, Yasuo
Journal of Nuclear Materials, 357(1-3), p.105 - 114, 2006/10
Times Cited Count:107 Percentile:98.82(Materials Science, Multidisciplinary)Electrorefining in the molten LiCl-KCl eutectic salt containing actinides(An) and rare-earths(RE) was conducted to recover up to 10 wt% An into liquid Cd cathode, which is much higher than the solubility of An in liquid Cd at the experimental temperature. In the saturated Cd cathode, An and RE were recovered to form a compound of the PuCd lattice, MCd
lattice, MCd . The separation factors of RE against Pu were defined as (RE/Pu in the Cd cathode)/(RE/Pu in the salt) and calculated for the saturated Cd alloy including MCd
. The separation factors of RE against Pu were defined as (RE/Pu in the Cd cathode)/(RE/Pu in the salt) and calculated for the saturated Cd alloy including MCd . The separation factors for the Cd cathodes were a little larger than the equilibrium values.
. The separation factors for the Cd cathodes were a little larger than the equilibrium values.
 F
F irradiated with two-frequency CO
irradiated with two-frequency CO laser lights
laser lightsYokoyama, Atsushi; Oba, Hironori; Hashimoto, Masashi; Katsumata, Keiichi; Akagi, Hiroshi; Ishii, Takeshi*; Oya, Akio*; Arai, Shigeyoshi*
Applied Physics B, 79(7), p.883 - 889, 2004/11
Times Cited Count:10 Percentile:42.05(Optics)Silicon isotope separation has been done utilizing the Infrared Multiphoton Dissociation of Si F
F irradiated with two-frequency CO
irradiated with two-frequency CO laser lights. The two-frequency excitation method improved the separation efficiency with keeping the high enrichment factors. For example, Si
laser lights. The two-frequency excitation method improved the separation efficiency with keeping the high enrichment factors. For example, Si F
F with the
with the 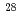 Si fraction of 99.4 % was obtained at 40.0 % dissociation of Si
Si fraction of 99.4 % was obtained at 40.0 % dissociation of Si F
F after the simultaneous irradiation of 100 pulses with 966.23 cm
after the simultaneous irradiation of 100 pulses with 966.23 cm photons (0.089 J/cm
photons (0.089 J/cm ) and 954.55 cm
) and 954.55 cm photons (0.92 J/cm
photons (0.92 J/cm ), while 1000 pulses were needed to obtain 99.0 % of
), while 1000 pulses were needed to obtain 99.0 % of 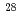 Si at 27.2 % dissociation in the case of single frequency irradiation at 954.55 cm
Si at 27.2 % dissociation in the case of single frequency irradiation at 954.55 cm (0.92 J/cm
(0.92 J/cm ). The single-step enrichment factors of
). The single-step enrichment factors of 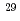 Si and
Si and 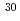 Si increased with increasing Si
Si increased with increasing Si F
F pressure. The reason for this enhancement has been discussed in terms of the rotational and vibrational relaxations by collisions with ambient gases.
pressure. The reason for this enhancement has been discussed in terms of the rotational and vibrational relaxations by collisions with ambient gases.
 solutions using
solutions using  -dimethyl-
-dimethyl- -diphenylpyridine-2,6-dicarboxyamide
-diphenylpyridine-2,6-dicarboxyamideShimada, Asako; Yaita, Tsuyoshi; Narita, Hirokazu; Tachimori, Shoichi; Kimura, Takaumi; Okuno, Kenji*; *
Solvent Extraction Research and Development, Japan, 11, p.1 - 10, 2004/04
The distribution ratio ( ) of Am(III) and lighter Ln(III) in the extraction with
) of Am(III) and lighter Ln(III) in the extraction with  -dimethyl-
-dimethyl- -diphenylpyridine-2,6-dicarboxyamide(DMDPhPDA) from HNO
-diphenylpyridine-2,6-dicarboxyamide(DMDPhPDA) from HNO solutions were determined. The D's increased with an increase of HNO
solutions were determined. The D's increased with an increase of HNO concentration. Additionally, separation of Am(III) from Ln(III) were confirmed for all the HNO
concentration. Additionally, separation of Am(III) from Ln(III) were confirmed for all the HNO concentration range (S.F.=(D
concentration range (S.F.=(D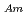 /D
/D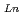 )). From 4 M HNO
)). From 4 M HNO solution with 0.5 M DMDPhPDA CHCl
solution with 0.5 M DMDPhPDA CHCl solution, the
solution, the  's of Am(III), Eu(III) and Nd(III) were 1.3, 0.25 and 0.24, respectively. This result suggests that Am(III) can be separated from Eu and Nd in the higher HNO
's of Am(III), Eu(III) and Nd(III) were 1.3, 0.25 and 0.24, respectively. This result suggests that Am(III) can be separated from Eu and Nd in the higher HNO concentration region than that has been reported so far. These
concentration region than that has been reported so far. These  's value and the S.F. were reproduced in the extraction from the metal concentration range from 10
's value and the S.F. were reproduced in the extraction from the metal concentration range from 10 M order to 10
M order to 10 M.
M.
Abe, Hitoshi; Usuda, Shigekazu; Takeishi, Hideyo; Tachimori, Shoichi
J. Liquid Chromatogr., 16(12), p.2661 - 2672, 1993/00
no abstracts in English
Journal of Radioanalytical and Nuclear Chemistry, 111(2), p.399 - 410, 1987/02
Times Cited Count:15 Percentile:79.7(Chemistry, Analytical)no abstracts in English
; ; *; ;
Journal of Radioanalytical and Nuclear Chemistry, 116(1), p.125 - 132, 1987/01
Times Cited Count:3 Percentile:38.12(Chemistry, Analytical)no abstracts in English
Fujie, Makoto; *; *; *
Journal of Nuclear Science and Technology, 23(4), p.330 - 337, 1986/00
Times Cited Count:46 Percentile:96.4(Nuclear Science & Technology)no abstracts in English
; Saito, Keiichiro; Shiba, Koreyuki
Journal of Nuclear Science and Technology, 20(5), p.439 - 440, 1983/00
Times Cited Count:53 Percentile:99.42(Nuclear Science & Technology)no abstracts in English